The battery acid chemical formula is H2SO4. This sulfuric acid is a strong electrolyte and is used in lead-acid batteries. When mixed with water, it forms an acidic solution that can corrode metal.
Battery acid is a corrosive substance that is used in lead-acid batteries. It is made up of a mixture of water and sulfuric acid. The chemical formula for battery acid is H2SO4.
This substance is highly corrosive and can cause serious damage to skinthe and eyes if it comes into contact with them. It is important to be very careful when handling battery acid and to wear proper protective gear if you are going to be working with it.
What Chemicals are in Battery Acid?
Most household batteries are acid-based. The acid is usually sulfuric acid, but can also be muriatic, phosphoric or nitric acids. The concentration of the acid solution varies by battery type; car batteries have a more dilute solution than do most household batteries.
The electrolyte in a lead-acid battery is made up of about 30 to 40 percent sulfuric acid and 60 to 70 percent water. In a fully charged cell, all the sulfuric acid is used up and, only water remains. When lead sulfate crystals form on the plates during discharge, some sulfuric acid is released back into the electrolyte.
That’s why it’s important to keep lead-acid batteries topped off with distilled water (not tap water) to maintain the proper level of sulfuric acid in the electrolyte. Muriatic (hydrochloric) acid is used in some car batteries, although not as commonly as it once was. Phosphoric acid has been used in experimental zinc-air storage batteries.
And concentrated nitric acid has been used as an electrolyte in early nickel-cadmium (NiCd) batteries and is still used today in some types of sealed NiCd rechargeable batteries designed for high power applications such as electric vehicles and cordless power tools..
What is Battery Acids Chemical Formula?
Battery acids are usually sulfuric or lead-based. The chemical formula for sulfuric acid is H2SO4, and the chemical formula for lead acid is PbO2.
What is the Acid in a Battery?
Batteries are devices that store energy and provide a current when needed. The first battery was invented in 1799 by Alessandro Volta, and since then the,y have become an essential part of our lives. Batteries power everything from our phones to our cars.
The acid in a battery is sulfuric acid. This acid is corrosive and can cause burns if it comes into contact with skinthe .The concentration of the acid in a battery varies depending on the type of battery, but it is typically around 30-40%.
Sulfuric acid is used in batteries because it is able to store large amounts of energy. When the battery is being used, the sulfuric acid reacts with the lead plates inside the battery, producing lead sulfate and water. This reaction releases electrons, which flow through the circuit and power whatever device is connected to the battery.
When the battery is not being used, the lead sulfate breaks down into lead and sulfate ions. The sulfuric acid then absorbed these ions, restoring it to its original state. This process can be repeated over and over again, allowing batteries to be reused many times.
What is the Chemical Name of the Battery?
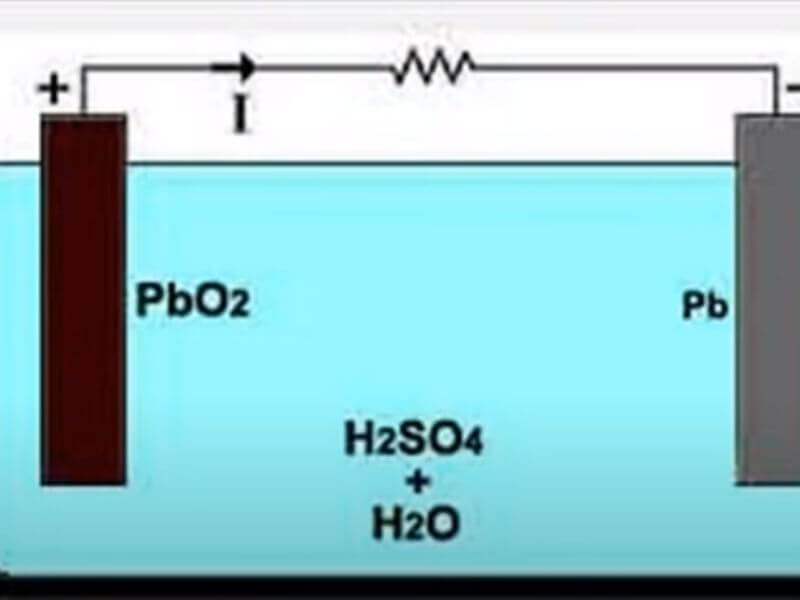
Batteries are devices that store chemical energy and convert it to electrical energy. The most common type of battery is the lead-acid battery, which consists of lead dioxide (PbO2) and sponge lead (Pb) electrodes in an electrolyte of sulfuric acid (H2SO4). When a lead-acid battery is discharged, the PbO2 electrode reacts with the H2SO4 electrolyte to form PbSO4 and water.
The Pb electrode does not react under normal conditions. During charging, the reverse reactions occur, converting PbSO4 and water back to PbO2 and H2SO4.
Battery Acid PH
When it comes to battery acid, there are a few things you should know:
| Number one | First, battery acid is highly corrosive and can cause serious damage to your skin and eyes. |
| Number two | Second, battery acid is also flammable, so it’s important to be careful when handling it. |
| Number three | Finally, battery acid is poisonous if ingested, so it’s important to keep it away from children and pets. If you come into contact with battery acid, flush the affected area with water for at least 15 minutes. If you get any in your eyes, seek medical attention immediately. |
| Number four | If you ingest battery acid, call poison control or go to the emergency room immediately. Battery acid is dangerous stuff, so be sure to handle it with care! |
Car Battery Acid Name

Car battery acid is a sulfuric acid solution used as an electrolyte in lead-acid batteries. It is a strong acid with a pH of 1.2-2.0. Battery acid is corrosive and can cause burns to the skin and eyes.
It is also dangerous if inhaled or swallowed.
Battery Acid Liquid
When most people think of battery acid, they think of the corrosive sulfuric acid found in car batteries. However, battery acid can also refer to any acidic solution used in a battery. This can include lead-acid, nickel-cadmium, nickel-metal hydride, and lithium-ion solutions.
Sulfuric acid is the most common type of battery acid. It is highly corrosive and can cause serious burns if it comes into contact with the skin. If ingested, it can be fatal.
Sulfuric Acid
Sulfuric acid is used in lead-acid batteries, commonly found in cars and other vehicles. Lead-acid batteries use a chemical reaction between lead and sulfuric acid to create an electrical current. The lead plates in the battery are coated with a layer of active material that reacts with sulfuric acid to create electrons. These electrons flow through a circuit to power whatever device is connected to the battery.
Nickel-Cadmium
Nickel-cadmium (NiCd) batteries use a similar chemical reaction between nickel and cadmium to create electricity. NiCd batteries are often used in small devices like handheld radios or toys because they are lightweight and have a long shelf life.
However, NiCd batteries are being phased out due to their environmental impact – cadmium is a toxic metal that can pollute soil and water when it leaches out of landfill sites where NiCd batteries are disposed of. Nickel-metal hydride (NiMH) batteries work similarly to NiCd batteries but use different metals – usually nickel and iron – in their electrodes. They are more environmentally friendly than NiCd batteries as they do not contain cadmium.
However, they tend to be less efficient than Li-ion batteries and have shorter lifespans.
Battery Acid Refill
If your car’s battery is running low, you may be tempted just to buy a new one. But with a little time and effort, you can easily refill it yourself with battery acid. Here’s what you’ll need:
- Safety goggles
- Rubber gloves
- Funnel
- Battery acid (you can purchase this at most auto parts stores)
- distilled water
Now follow the step by step guideline:
First Step
Battery acid is corrosive and can cause serious skin and eye damage, so it’s important to take precautions when handling it.
Second Step
Use the funnel to pour the battery acid into the cell until it reaches the fill line. Be careful not to overfill!
Final Step
Once all the cells are filled, top off with distilled water until the liquid level reaches the top of the battery’s plates. Distilled water is necessary because regular tap water contains minerals that can damage the battery cells.There are also different minerals in batteries.
Your battery is now refilled and ready to go.
Battery Acid Vs Distilled Water
When it comes to battery acid and distilled water, there are a few key differences that you should be aware of:
| Battery Acid | Distilled Water |
| For starters, battery acid is much more corrosive than distilled water. This means that it can cause serious damage to any surfaces it comes into contact with, including your skin. | In contrast, distilled water is not nearly as corrosive and will not cause immediate damage to your skin. However, over time, repeated exposure to distilled water can lead to dryness and irritation. |
| Battery acid has a pH level of around 1. | While distilled water has a pH level of 7. |
| This means that battery acid is extremely acidic. | While distilled water is only slightly acidic |
So which one should you use?
If you need to clean up a small spill of battery acid, then using distilled water is perfectly fine. Just be sure to rinse the area thoroughly with fresh water afterwards. However, suppose you’re dealing with a larger spill or need to clean something that’s been heavily contaminated with battery acid. In that case, it’s best to call a professional hazmat team for assistance.
Battery Acid on Skin
If you have battery acid on your skin, it’s important to act quickly.
Battery acid is highly corrosive and can cause serious burns. Here’s what you should do:
1. Flush the affected area with cool water for at least 15 minutes.
2. Apply a sterile bandage or clean cloth to the area.
3. Seek medical attention if the burning sensation persists or if you see any signs of infection, such as redness, swelling, or pus drainage.
How to Make Battery Acid
Most people think of battery acid as a dangerous and corrosive substance. However, did you know that you can make your battery acid at home?
Here’s how:
| Step one | Start by mixing equal parts of sulfuric acid and water in a container |
| Step two | Next, slowly add more sulfuric acid to the mixture until it reaches a concentration of about 70%. |
| Step three | Once the desired concentration is reached, add a small amount of potassium permanganate to the mixture and stir well. |
| Step four | Finally, allow the mixture to sit for 24 hours before using it as battery acid |
Where to Buy Sulfuric Acid for Batteries?
You can check a few places if you’re looking for sulfuric acid for batteries. Your local hardware store or battery retailer is a good place to start. You can also find it online, but check the seller’s reputation and reviews before making a purchase.
When buying sulfuric acid, be sure to get the right concentration. For most applications, concentrated (93%) sulfuric acid is what you need. Be careful when handling this chemical, as it is extremely corrosive and can cause serious burns.
Always wear gloves and protective eyewear when working with it.
What Are the Different Types of Potential Energy?
There are several different types of potential energy, including nuclear energy potential. This form of energy is stored in the nucleus of an atom and can be released through nuclear reactions. It is a highly concentrated and powerful source of energy, making it a topic of great interest and research in the field of physics and energy production.
What are the benefits of using Lithium-iron Phosphate (LFP) batteries compared to batteries with acid chemical formulas?
When it comes to energy storage, lfp battery technology offers numerous benefits over traditional acid-based batteries. LFP batteries are known for their longer lifespan, higher energy density, and improved safety. This makes them an attractive option for various applications, including electric vehicles and renewable energy storage systems.
What Other Chemicals are Found in Batteries Besides Battery Acid?
Besides battery acid, various other chemicals are found in batteries. These battery chemicals, also known as electrolytes, include potassium hydroxide and sulfuric acid. These substances facilitate the movement of electrons between the battery’s electrodes, enabling the efficient flow of electrical energy. Additionally, batteries may also contain other elements such as nickel, lithium, and cadmium, depending on their type and composition.
End Note
If you’re looking for the battery acid chemical formula, you’ve come to the right place. This article will give you a rundown of what battery acid is and its chemical makeup. Battery acid is an electrolyte solution that is found in lead-acid batteries.
It is made up of water and sulfuric acid and has a specific gravity of 1.21. The sulfuric acid concentration in battery acid can vary from 30% to 60%. The main function of battery acid is to provide electrons to the lead plates in the battery to produce an electrical current.
A chemical reaction occurs when the lead plates are submerged in sulfuric acid, producing lead sulfate and hydrogen gas. When the battery is charged, the lead sulfate is converted back into the lead and sulfuric acid. This process repeatedly happens as long as the battery has enough power remaining.
So there you have it; that’s the basic rundown of what goes into making up battery acid and how it works!
References:
