Dry cells produce an electric current. This is done by a process called electrolysis. For this to happen, an electrolyte must be present in the cell.
The electrolyte is what provides the ions that are necessary for the reaction to take place. The most common electrolyte used in dry cells is sulfuric acid.
Dry cell batteries are a type of electrochemical battery. Dry cells use a metal oxide as the anode and a carbon rod as the cathode. The anode is usually made of zinc, while the cathode can be made from any number of materials, including carbon, manganese dioxide, or copper oxide.
A dry cell produces an electric current through a chemical reaction between the anode and cathode. This reaction creates electrons, which flow from the anode to the cathode through an external circuit. Dry cells are used in many standard devices, such as flashlights, radios, and portable electronic devices.
They are also used in some vehicles, although most modern cars use wet-cell batteries. Dry cells have a few advantages over wet cell batteries. They are less likely to leak, and they can be stored for long periods of time without being damaged.
However, dry cells also have some disadvantages. They typically have shorter lifespans than wet cell batteries and can be more expensive to replace.
A dry Cell is an Example of an Electrochemical Cell
A dry cell is an electrochemical cell that uses a solid electrolyte instead of a liquid one. The lead-acid battery is the most common dry cell type, consisting of a lead anode and a lead dioxide cathode, with sulfuric acid as the electrolyte. Other types of dry cells include zinc-carbon batteries and nickel-cadmium batteries.
How Does a Dry Cell Battery Work?
A dry cell battery is an electrical energy storage device that uses an electrolyte paste instead of a liquid electrolyte. The paste is a mixture of sulfuric acid and lead dioxide, which react to create an electrical current. The reaction between sulfuric acid and lead dioxide produces heat, so the battery must be vented to release the gas. Click here to learn more about electric energy and how it converts into chemical energy.
Dry cell batteries are used in many everyday devices, such as flashlights, portable radios, and smoke detectors. They are also used in some larger items like cars and boats. Dry cell batteries are cheaper than other types of batteries and last longer.
What is a Dry Cell in Chemistry?
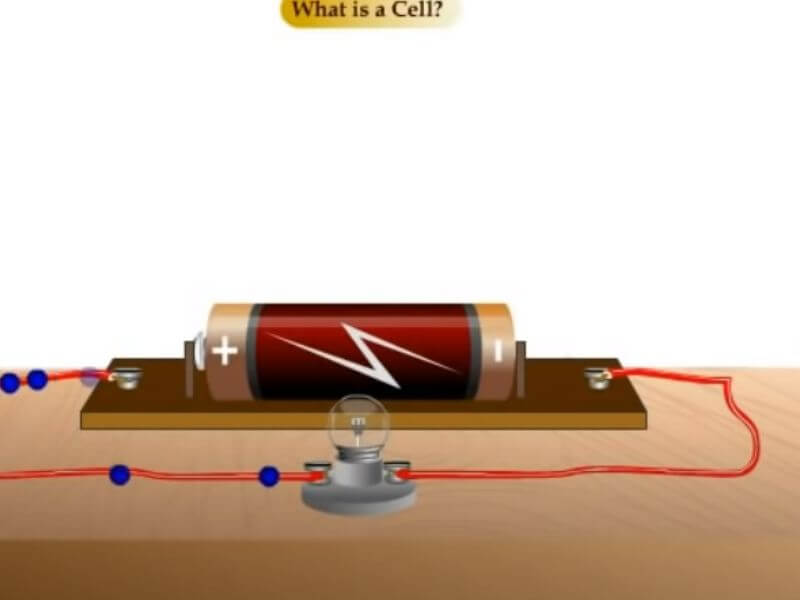
A dry cell is an electrochemical cell that uses an electrolyte, not in liquid form. The most common type of dry cell is the lead-acid battery. Lead-acid batteries are typically used in cars and other vehicles.
Other types of dry cells include zinc-carbon batteries and lithium-ion batteries.
Uses of Dry Cell
A dry cell is a type of electrolytic cell where an electrolyte is in a paste form or, in other words, it is solid. The term “dry cell” is usually used when referring to lead-acid batteries. A lead-acid battery has a positive plate of lead dioxide and a negative plate of spongy lead.
The electrolyte between these two plates is sulfuric acid in water. When the battery produces electricity, the sulfuric acid attacks the lead oxide on the positive plate, turning it into lead sulfate and water. This process also happens on the negative plate, but with the formation of lead instead of lead sulfate.
Dry Cell is Also Known As!
A dry cell is an electrical battery that uses an electrolyte paste instead of a liquid. The paste is usually made from potassium hydroxide and zinc oxide. Dry cells are used in many portable electronic devices, such as radios, flashlights, and laptop computers.
Dry Cell Electrolyte

A dry cell electrolyte is a type of electrolyte used in batteries. It comprises a solid material, such as an ion-exchange resin, and a liquid. The solid material acts as a separator between the positive and negative electrodes, while the liquid conducts ions between them.
This type of electrolyte has several advantages over other types, including:
| Increased safety | Since the solid material prevents direct contact between the electrodes, there is less risk of short circuits or fires. |
| Longer shelf life | Since there is no water to evaporate, dry cell batteries can be stored for longer periods of time without losing their charge. |
| Increased power density | Because the ions have a shorter distance to travel through the solid material, more energy can be stored in a given volume than other types of electrolytes. |
Does Dry Cell Produce Electricity?
The dry cell does produce electricity, but the process is not as simple as one might think. For a dry cell to generate electricity, a chemical reaction must occur between the anode and cathode materials.
- This reaction produces electrons, which flow through an external circuit from the anode to the cathode.
- The flow of electrons produces an electric current, which can be used to power electrical devices. The chemical reaction that occurs in the dry cell is known as electrolysis.
- During electrolysis, molecules of the anode material are oxidized, releasing electrons into the external circuit.
At the same time, molecules of the cathode material are reduced, accepting electrons from the external circuit. The overall result is a transfer of electrons from the anode to the cathode, producing an electric current. Dry cell batteries are commonly used in portable electronic devices such as flashlights and radios.
They are also used in some industrial applications where a reliable source of electricity is required, but there is no access to mains power.
What Kind of Current Does a Dry Cell Produce?
A dry cell is a battery that uses an electrolyte paste instead of a liquid. The paste is made up of sulfuric acid and lead dioxide, which react to create an electrical current. This type of battery is often used in flashlights and other portable devices.
Final Thought
A dry cell is a battery that uses an electrolyte paste instead of a liquid. The paste is made up of powdered zinc and carbon, which are separated by a thin layer of paper or plastic. The electrolyte paste conducts electricity between the two materials, allowing current to flow through the circuit.
Dry cells are used in various devices, including flashlights, portable radios, and cordless power tools.
Used Resources:
