Lithium ion batteries are one of the most popular types of batteries on the market today. They are used in a wide variety of devices, from cell phones to laptops. One of the key components of a lithium ion battery is the separator.
The separator is a thin sheet of material that separates the positive and negative electrodes in the battery. It is an important part of the battery because it prevents the electrodes from coming into contact with each other, which could cause a short circuit.

There are many different types of batteries, and each has its own benefits and drawbacks. One type of battery that is becoming increasingly popular is the lithium ion battery. Lithium ion batteries have a number of advantages over other types of batteries, but one of the most notable is their high energy density.
This means that they can store more energy than other types of batteries, making them ideal for use in portable electronic devices. One important part of a lithium ion battery is the separator. The separator is a thin layer of material that separates the positive and negative electrodes in the battery.
It is essential to prevent the two electrodes from coming into contact with each other, as this would cause a short circuit and potentially damage the battery. The separator also needs to be permeable to ions so that they can flow between the electrodes during charging and discharging.
What is the Separator Used in Batteries?
Batteries are one of the most common power sources in our world today. They come in all shapes and sizes, from tiny watch batteries to the massive lead-acid batteries used in cars and trucks. But what exactly is a battery?
A battery is basically two or more electrochemical cells connected together. Each cell has three parts: an anode (negative electrode), a cathode (positive electrode), and an electrolyte (the stuff in between). When you connect a battery to something like a light bulb, it creates a circuit.
Electrons flow from the negative anode through the electrolyte to the positive cathode. This flow of electrons produces electricity, which powers the light bulb. The separator is a very important part of the battery.
It helps keep the electrodes from touching each other directly, which would cause them to short out and stop working. The separator also allows electrons to flow freely between the electrodes while keeping the electrolyte contained.
Lithium-Ion Battery Separator Thickness
Lithium-ion battery separator thickness is an important factor in the performance and safety of lithium-ion batteries. The separator is a thin film that separates the positive and negative electrodes of the battery, and its main function is to prevent short circuits. The thickness of the separator influences several key characteristics of the battery, including capacity, voltage, power density, energy density, and safety.
A thinner separator allows for a higher charge/discharge rate and a higher energy density (the amount of energy that can be stored in a given volume), but it also decreases the safety margin against potential shorts. As such, finding the optimal balance between these conflicting properties is critical for designing safe and high-performance lithium-ion batteries. There are two main types of separators used in lithium-ion batteries: microporous and nonporous.
Microporous separators are made from polyolefin materials like polyethylene or polypropylene, and they have tiny pores that allow ions to pass through while still preventing contact between the electrodes. Nonporous separators are made from ceramic materials like alumina or silicon oxide, and they do not have pores; instead, they rely on an electrical barrier to keep the electrodes apart. Both types of separators have their advantages and disadvantages.
Microporous separators are less expensive to produce than nonporous ones, but they are also more prone to degradation over time due to electrolyte leakage through their pores. Nonporousseparators are more expensive to produce but don’t degrade as quickly; however, they can become electrically resistive over time if there is any contamination on their surfaces. The optimum thickness for a given application depends on many factors, including electrode composition, charging/discharging rates, operating temperature range, etc.
Lithium-Ion Battery Separator Manufacturing Process
Lithium-ion batteries are one of the most popular types of batteries on the market today. They are used in a variety of applications, from cell phones to laptops to electric vehicles. One of the key components of a lithium-ion battery is the separator.
The separator is a thin sheet of material that sits between the anode and cathode of the battery, and its primary purpose is to prevent electrical shorts between these two electrodes. There are several different materials that can be used for battery separators, but the most common is polyethylene (PE). PE separators are made via a process called extrusion, where molten PE is forced through a die to create thin sheets.
This process can be performed using either linear low-density polyethylene (LLDPE) or high-density polyethylene (HDPE). Once the desired thickness has been achieved, the sheets are then cooled and cut into rolls. These rolls are then shipped off to battery manufacturers, where they will be used in the production of lithium-ion batteries.
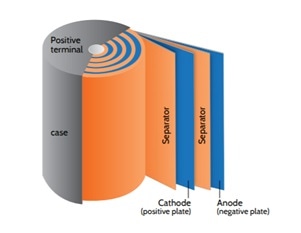
Types of Battery Separators
The separator is a very important component in the lead acid battery. It acts as a physical barrier between the positive and negative electrodes, allowing electrical current to flow while preventing physical contact and chemical reactions between the two electrodes. The three main types of separators used in lead acid batteries are microporous, glass mat, and gel.
Microporous separators are made of thin sheets of porous material, usually polyethylene or polypropylene. The pores in the material are small enough to prevent electrons from flowing between the electrodes, but allow ions to pass through freely. This type of separator is typically used in automotive batteries where high currents are required.
Glass mat separators are also made from thin sheets of material, but instead of being porous, they are coated with a fine layer of glass fibers. The glass fibers prevent both electrons and ions from passing through, but allow electrolytes to flow freely. Glass mat separators are often used in deep cycle batteries where long life and low maintenance are required.
Gel separators are similar to glass mat separators, but instead of being coated with glass fibers, they are impregnated with silicone gel. This gel completely surrounds the electrodes and prevents any movement of electrons or ions between them. Gel separators offer the longest life and lowest maintenance requirements of all three types, but they also require higher manufacturing costs.
Role of Separator in Battery
Separators are one of the most important components in batteries. They play a key role in ensuring safety and performance by keeping the positive and negative electrodes apart. Separators are made from materials that are electrically insulating but mechanically strong, so they can withstand being repeatedly charged and discharged.
Batteries work by storing energy in an electrochemical reaction between the positive and negative electrodes. The separator allows this reaction to take place while preventing the two electrodes from coming into contact with each other, which would cause a short circuit. When a battery is being charged, electrons flow from the negative electrode to the positive electrode through the separator.
This creates an electrostatic potential difference between the two electrodes, which drives the chemical reaction that stores energy in the battery. During discharge, the process is reversed and electrons flow from the positive electrode to the negative electrode through an external circuit. This causes a chemical reaction that releases energy, which can be used to power devices such as electric motors.
The separator also plays an important role in preventing self-discharge, which is when a battery loses its charge even when it’s not being used. This happens because some of the chemicals involved in the electrochemical reactions spontaneously react with each other, causing electrons to flow between the electrodes without going through an external circuit. If there wasn’t a separator, these reactions would cause a short circuit and discharge all of the stored energy in the battery very quickly.
By keeping the electrodes separated, self-discharge is slowed down significantly.
The electrolyte in Lithium-Ion Battery
In a lithium-ion battery, the electrolyte is a critical component that plays several important roles. It helps to shuttle charged particles between the anode and cathode, acts as a barrier to prevent the escape of those charged particles, and also helps to keep the battery stable during charging and discharging. The electrolyte used in lithium-ion batteries is typically a liquid or gel, and it contains a mix of organic solvents and lithium salts.
The most common type of salt used is lithium hexafluorophosphate (LiPF6), but other salts such as LiClO4 and LiBOB can also be used. The choice of electrolyte has a big impact on the performance of the battery. For example, using a high concentration of LiPF6 can help to improve charge/discharge efficiency, but it can also lead to increased degradation of the electrodes over time.
Finding the right balance is key to optimizing battery performance.
Ceramic Separator for Lithium-Ion Battery
Lithium-ion batteries are used in a wide variety of electronic devices, from cell phones to laptops. However, these batteries can be expensive to replace. A ceramic separator can extend the life of a lithium-ion battery, making it a valuable investment for anyone who uses these devices regularly.
A ceramic separator is a thin sheet of material that is placed between the positive and negative electrodes of a lithium-ion battery. This separator helps to prevent the electrodes from coming into contact with each other, which can cause short circuits and other problems. A good quality separator will also help to keep the electrolyte in the battery stable, preventing it from becoming too hot or cold.
There are many different types of ceramic separators on the market, so it is important to choose one that is compatible with your specific type of battery. You should also make sure that the separator you select has been tested and approved by a reputable third party. With proper care and maintenance, your lithium-ion battery should last for many years with a ceramic separator in place.
Separator in Battery

The separator in a battery is an important component that keeps the positive and negative electrodes from coming into contact with each other. This prevents a short circuit from occurring, which could lead to a fire or explosion. The separator also allows electrons to flow between the electrodes, providing the current that powers your devices.
FAQs
What is the Separator Made Of?
The separator is typically made of plastic or metal. The most common type of separator is the plastic egg crate, which is used in many households. Metal separators are also available, but they are less common.
What are Lithium-Ion Battery Separators Made Of?
Lithium-ion battery separators are made of a thin, porous material that allows ions to flow between the anode and cathode while preventing electrons from flowing. The most common material used for lithium-ion battery separators is polyethylene, which is a plastic. Other materials that have been used include polypropylene and cellulose.
Bottom Line
Lithium ion batteries are used in many electronic devices, including cell phones and laptops. The separator is a critical component of the battery, as it keeps the positive and negative electrodes from coming into contact with each other. If the electrodes were to touch, the battery would short circuit and could potentially catch fire.
The separator is made of a thin film that is permeable to lithium ions, but not electrons. This allows lithium ions to flow between the electrodes while preventing electrical current from flowing through the battery.
