An electrolyte is a substance that produces an electrically conducting solution when dissolved in a polar solvent, such as water. The dissolved electrolyte separates into cations and anions, which disperse uniformly throughout the solution. A battery is a device that converts chemical energy into electrical energy.
The most common type of battery used today is the lead-acid battery. Ion batteries are a type of battery that uses electrolytes to create an electric current. The most common electrolyte used in ion batteries is lithium, but other options include sodium, potassium, and magnesium. Each type of electrolyte has its own benefits and drawbacks, so choosing the right one for your application is important.
Lithium-ion batteries are the most popular type of ion battery on the market today. They offer a high energy density and long life span, making them ideal for use in portable electronic devices like laptops and cell phones. However, lithium-ion batteries can be expensive, and they can be dangerous if not properly handled (for example, if the battery is punctured or overheated).
Sodium-ion batteries are another option for ion batteries. They have a lower energy density than lithium-ion batteries, but they are cheaper to produce and safer to handle. Sodium-ion batteries also have a shorter life span than lithium-ion batteries, so they are not typically used in applications where long-term reliability is required.
Potassium-ion batteries are similar to sodium-ion batteries in terms of cost and safety, but they have a higher energy density than both sodium and lithium-ion batteries. Potassiumionbatteries also have a longer life span than sodium-ion batteries, making them a good choice for applications where reliability is important. However, potassium ion batteries can be difficult to find on the market due to their relatively new technology.
Magnesiumionbatteries are the newest type of electrolyte on the market. They offer several advantages over other types of electrolytes: they are non-flammable, non-toxic, and have a very high energy density. However, magnesium ion batteries are also expensive and tend to degrade quickly when exposed to heat or light.
As such, they are not yet widely used.
Use of Electrolyte in Battery
Batteries are devices that store energy and convert it into electrical energy. The most common type of battery is the lead-acid battery, which uses a chemical reaction between lead and acid to create electrical energy. This type of battery typically contains a solution of sulfuric acid and water.
When a lead-acid battery is discharged, the sulfate ions in the acid react with the lead electrodes to produce lead sulfate. During charging, the reverse process occurs and the lead sulfate is converted back into sulfuric acid. This chemical reaction produces electrical energy, which can be used to power electronic devices.
The use of electrolytes in batteries helps to improve their performance by providing a conductive medium for the reactions between the electrodes and the electrolyte. Electrolytes also help to prevent corrosion of the electrodes and provide a cooling effect for batteries that generate heat during operation.
Lithium-Ion Battery Electrolyte Manufacturers
Lithium-Ion Battery Electrolyte Manufacturers Lithium-ion batteries are one of the most popular types of batteries on the market today. They are used in everything from cell phones to laptops to electric cars.
And, as the demand for lithium-ion batteries continues to grow, so does the demand for lithium-ion battery electrolyte manufacturers. There are a number of different companies that manufacture lithium-ion battery electrolytes. Some of the more well-known manufacturers include BASF, Dow Chemical, and LITHIUM POWER Inc.
However, there are many other companies that also manufacture lithium-ion battery electrolytes. Each manufacturer has its own unique formula for its lithium-ion battery electrolytes. This is what gives each manufacturer its own unique properties and performance characteristics.
As such, it is important to choose a manufacturer that can provide the specific type of electrolyte that you need for your application. When choosing a lithium-ion battery electrolyte manufacturer, there are a few things to keep in mind.
| First | You need to make sure that the company is able to produce high quality products. |
| Second | You need to make sure that the company has a good reputation. And third, you need to make sure that the company is able to meet your specific needs. |
Role of Electrolyte in Lithium-Ion Battery
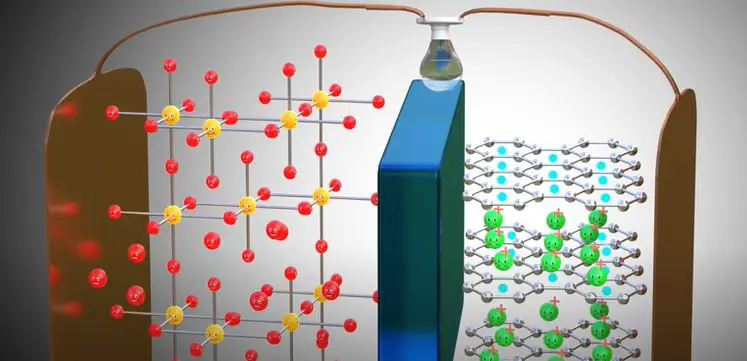
Lithium-ion batteries are among the most popular types of batteries for portable electronics, with a high energy density and long life span. However, they can be dangerous if not used correctly, as they are highly reactive and can catch fire or explode if damaged. One of the key components of a lithium-ion battery is the electrolyte, which consists of a lithium salt in an organic solvent.
The electrolyte is responsible for carrying charge between the anode and cathode of the battery, and also helps to prevent the formation of dendrites on the anode. If the electrolyte is too concentrated, it can cause problems such as dendrite growth, reduced capacity, and increased self-discharge. On the other hand, if it is too dilute, it can lead to poor conductivity and reduced power output.
Therefore, it is important to use the correct concentration of electrolytes in order to maintain optimal performance from your lithium-ion battery.
Best Electrolyte for Battery
Batteries are one of the most important parts of our electronic devices. They provide the power that we need to use our phones, laptops, and other devices. Without batteries, we would be stuck in the dark ages!
There are many different types of batteries, but they all have one thing in common: they need electrolytes to function. Electrolytes are a type of salt that helps to conduct electricity. Without electrolytes, batteries wouldn’t be able to store or release energy.
So, what is the best electrolyte for batteries? The answer depends on the type of battery you’re using. For example, lead-acid batteries use sulfuric acid as their electrolyte while lithium-ion batteries use lithium salt.
No matter what type of battery you’re using, it’s important to make sure that your electrolyte levels are topped off. This will help to ensure that your battery is able to perform at its best.
Lithium-Ion Battery Electrolyte Review
Lithium-Ion Battery Electrolyte Review Batteries are a critical part of our lives, providing power to everything from our cell phones to our cars. And while there are many different types of batteries, lithium-ion batteries have become increasingly popular in recent years due to their high energy density and long life.
But what exactly is a lithium-ion battery? And how does it work? In this blog post, we’ll take a closer look at the science behind these amazing devices and learn about the electrolyte that makes them tick.
A lithium-ion battery is made up of two electrodes (usually made of metal), separated by an electrolyte. This electrolyte is typically a liquid or gel, and it contains lithium ions. When the battery is charged, these ions flow from the negative electrode to the positive electrode.
When the battery is discharged, the process is reversed and the ions flow back to the negative electrode. The key to a lithium-ion battery’s high energy density is its ability to store large amounts of these lithium ions in the electrodes. And that’s where the electrolyte comes in.
The electrolyte must be able to not only transport these ions between electrodes but also keep them stable during charging and discharging cycles. There are many different types of electrolytes used in lithium-ion batteries, but one of the most popular is LiPF6 (lithium hexafluorophosphate). This compound has several advantages over other electrolytes: it has a high solubility for lithium ions, it doesn’t decompose at high voltages, and it has good thermal stability.
However, LiPF6 does have some drawbacks: it’s expensive, corrosive, and toxic. For this reason, researchers are constantly searching for alternative electrolytes that can address some of these issues while still maintaining good performance.
Lipf6 Electrolyte
Lipf6 is an electrolyte that is used in batteries. It is a liquid that contains lithium and fluorine atoms. The lithium atoms are responsible for carrying the electrical charge, while the fluorine atoms help to stabilize the lithium ions.
Lipf6 is a very stable compound, which makes it ideal for use in batteries. However, it is also corrosive and can damage battery cells if it leaks out of the battery.
Disadvantages of Lithium-Ion Battery
Lithium-ion batteries are one of the most popular types of rechargeable batteries on the market today. They are used in everything from cell phones and laptops to electric vehicles and grid storage applications. While lithium-ion batteries have many advantages over other types of batteries, they also come with some disadvantages.
Cost
One of the biggest disadvantages of lithium-ion batteries is their cost. Lithium-ion batteries are significantly more expensive than other types of rechargeable batteries, such as lead acid or nickel metal hydride batteries. This can make them prohibitively expensive for some applications.
Can Be Dangerous if Not Used Properly
Another disadvantage of lithium-ion batteries is that they can be dangerous if not used properly. Lithium-ion batteries can catch fire if they are damaged or improperly charged. This has led to a number of high-profile incidents, such as the infamous Galaxy Note 7 fiasco.
Relatively Short Lifespan
Lithium-ion batteries have a relatively short lifespan compared to other types of rechargeable batteries. They typically only last for around 500 charge cycles before needing to be replaced, while lead acid batteries can last for thousands of cycles.
Lithium-Ion Battery Uses
Lithium-ion batteries are used in a wide variety of devices, from computers to cell phones to hybrid cars. They offer a number of advantages over other types of batteries, including a longer lifespan and higher energy density. Lithium-ion batteries can also be charged more quickly than other types of batteries, making them ideal for use in devices that require frequent recharging.

Which Electrolyte is Best for Battery?
There are a few electrolytes that can be used in batteries, but the most common and best electrolyte for batteries is potassium hydroxide. This electrolyte has a high ionic conductivity, which means it can easily carry electrical current between the anode and cathode of a battery. Additionally, potassium hydroxide is non-reactive, so it won’t corrode the battery’s electrodes over time.
How Much Electrolyte is Used in a Lithium Ion Battery?
Lithium-ion batteries are used in a wide variety of devices, from cell phones to laptops. They are also used in some electric vehicles. The amount of electrolyte used in a lithium-ion battery depends on the size and capacity of the battery.
A small battery may only use a few grams of electrolyte, while a large battery can use up to several kilograms.
Which is the Electrolyte Used in Li-Ion Battery MCQ?
Lithium-ion batteries are powered by a chemical reaction between lithium and oxygen. The electrolyte used in these batteries is typically a mixture of LiClO4 and LiPF6 in an organic solvent such as ethylene carbonate. When the battery is discharged, lithium ions flow from the positive to the negative electrode where they react with oxygen to form lithium peroxide.
During charging, the reverse reaction takes place and lithium ions are oxidized back to their original state.
Why Electrolyte is Used in Battery?
The electrolyte is a key component in batteries, both lead-acid and lithium-ion. In a lead acid battery, the electrolyte is sulfuric acid, which reacts with the lead electrodes to create an electrical current. In a lithium-ion battery, the electrolyte is usually a mixture of lithium salts dissolved in an organic solvent.
These reactions allow electrons to flow between the electrodes, creating an electrical current that can be used to power devices. The main reason why electrolyte is used in batteries is that it helps to facilitate the flow of electrons between the electrodes. This flow of electrons is what creates an electrical current, which can then be used to power devices.
Electrolytes also help to protect the electrodes from corrosion and degradation.
The Bottom Line
There are three electrolytes commonly used in ion batteries: lithium, sodium, and potassium. Each of these electrolytes has its own advantages and disadvantages. Lithium is the lightest of the three, making it ideal for use in small devices such as cell phones.
However, it is also the most expensive. Sodium is heavier than lithium but cheaper, making it a good choice for larger devices such as electric vehicles. Potassium is the heaviest of the three electrolytes but has the advantage of being abundant and inexpensive.
